|
|
|

|
The ideas from section S-7 are reviewed in what follows next. The rest of the section is a qualitative discussion of all key processes involved in the practical use nuclear energy. A Review of Nuclear StructureThe way the Sun generates its energy helps understand the way a nuclear power station does so. The two processes are however quite different. Here some facts about the way protons and neutrons combine to form nuclei, as covered in section S-7 about the Sun:
In summary, then: iron nuclei are the most stable ones, and the best sources of energy are therefore nuclei as far removed from iron as possible. One can combine the lightest ones--nuclei of hydrogen (protons)--to form nuclei of helium, and that is how the Sun gets its energy. Or else one can break up the heaviest ones--nuclei of uranium--into smaller fragments, and that is what nuclear power companies do.
How many Protons, how many Neutrons?As already noted, protons and neutrons (jointly called "nucleons") are intrinsically similar, and can convert into each other, absorbing or emitting an electron to maintain proper electric charge. What determines how many of each are found in a nucleus?The nuclear forces apparently prefer equal numbers of each kind, and light nuclei--helium, carbon, nitrogen, oxygen--usually maintain a 50:50 ratio, although nuclear variants ("isotopes") with small deviations from equality may exist and may be stable. In heavier nuclei, because of the electric repulsion between protons, this equality no longer holds. Imagine a nucleus with 56 nucleons, and suppose we could choose how many of this total would be neutrons and how many protons. What would be the most stable combination? Choosing 28 of each kind might provide the most stable nuclear binding, but that is offset by the energy required to hold in close quarters 28 positive protons. So nature compromises: the preferred combination--the nucleus of the most common form of iron--has 30 neutrons but only 26 protons. As nuclei get heavier, the fraction of protons drops still further--about 45% in mid-range nuclei, and less than 40% in the heaviest ones, those of uranium. Ordinary uranium ("U-238") has 92 protons but 146 neutrons, for a total of 238 nucleons. As will be seen, this gradual change in the proton/neutron ratio is essential to the nuclear chain reaction.
Nuclear FissionUranium nuclei in nature are unstable. Each of their 92 protons repels the rest, and sooner or later (half of them within 4.5 billion years) they eject a positive fragment, an "alpha particle" which is another term for a rapidly moving nucleus of helium. Almost all the helium atoms we extract from natural gas and from rocks--for filling balloons and for other uses--were originally created as alpha particles. But there exists a way of speeding up this break-up, by exposing the material to free neutrons.
Such an attraction releases energy. If the nucleus is heavy and unstable, like that of uranium, adding energy destabilizes it even more, to where it may break up immediately. The interesting thing about such a break-up is the spectacular way in which it sometimes happens, as was discovered by Hahn and Meitner in 1939. Instead of breaking off a little chip, a helium nucleus containing less than 2% of the mass, the entire nucleus splits into 2 comparable fragments, typically containing 1/3 and 2/3 of the mass. This process is called nuclear fission and besides the speed with which it can take place, it has at least two other remarkable features:
Ordinarily, such an adjustment calls for the conversion of some neutrons into protons (plus emitted electrons, "beta rays"), a process known as "beta radioactivity." Such an adjustment does in fact occur, making such fragments fiercely radioactive, and turning their disposal into a major issue for the nuclear power industry. But at first, when the nucleus breaks up, the fragments are too unstable for such a gradual process. A quicker adjustment is needed, and the fragments achieve it by each emitting one or sometimes more free neutrons. The Chain ReactionOn the average, about two neutrons are released this way per fission event. But it takes only one neutron to initiate another fission! Thus if fissionable nuclei are so densely packed that each neutron is bound to produce a new fission, the number of fission events quickly multiplies: 2, 4, 8, 16, 32, 64, 128... Since the energy release is proportional to the rate of fission, it also grows--very quickly! This chain reaction is what makes a nuclear bomb (or "atomic bomb") function. The material with nuclei prone for fission--usually plutonium, an artificial heavy elements with 94 protons--must be compressed tightly and at the appropriate moment, exposed to a blast of neutrons. A variety of tricks, all of them top secret, is used to make sure that at least an appreciable fraction of its atoms undergoes fission before the whole thing blows apart. Commercial nuclear power is produced somewhat differently, in a more controlled fashion. The fuel is uranium 235 (U-235)--a variant ("isotope") with 92 protons but only 143 neutrons, not 146, an odd number which makes it less stable. Natural uranium consists mostly of U-238, and it can absorb a neutron without undergoing fission (it ultimately turns into plutonium). U-238 therefore will not support a chain reaction. However, 0.7% of uranium is U-235, which can fission as soon as it absorbs a neutron. |
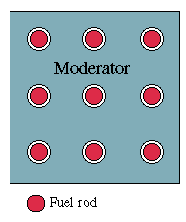 By using a clever design, one can actually build a reactor fueled by natural uranium. The trick is to form the fuel into rods, and put between them some material ("moderator") which slows down neutrons but does not absorb them, e.g. pure carbon or "heavy water" containing the heavy isotope of hydrogen. Neutrons produced in a rod generally escape into the moderator, and by the time they hit another rod, they are moving very slowly: such slow neutrons are gobbled up much more avidly by U-235 than by U-238, so that even in a rod containing only 0.7% U-235, the U-235 atoms make most of the "catches. " The Critical Mass |
|
It should be added that many neutrons are also lost--escaping from the edges of the reactor into the surrounding material, or being absorbed inside it by the " wrong " nuclei, the ones which do not undergo fission. In fact, a reactor needs to be carefully designed to sustain a chain reaction in the first place: but it can be done. From the beginning, complex and very expensive methods were devised to separate U-235 or to enrich its percentage past 0.7%. Today all commercial power reactors use enriched fuel, which makes the design of reactors easier and more controllable. With enriched fuel, ordinary water can serve as moderator, and it is even feasible to combine moderator and fuel, dissolving some uranium compound in water which acts both as moderator and as a distributor of heat. Such a reactor--or a chunk of plutonium--will not support a chain reaction if it is too small. If the amount of fissionable material is less than a critical mass, the average fission occurs too close to the surface. Even though (say) 2 neutrons are produced in each fission, on the average 1.2 of them escape to the outside before hitting another nucleus, leaving only 0.8 neutrons to continue the process, whereas one or more are needed. When processing nuclear fuel, or reprocessing fuel rods, it is therefore essential to work only with small quantities to prevent any accidental chain reaction. On 30 September 1999, at the nuclear processing plant in Tokaimura, Japan, workers thought they would save time by combining several batches of a solution of uranium. With a flash of blue light, a chain reaction began, giving three workers very bad doses of radiation and lasting 18 hours. After 3 months one worker died (in spite of extreme measures), one was discharged from the hospital and one is still (as of 12/99) in intensive care. A detailed report on the accident ("What Happened in Tokaimura?") appeared in "Physics Today", December 1999, p. 52-4. A similar accident occured in the US in the 1950s, when a worker extracting plutonium from a solution in one liquid into another took a shortcut and combined several batches. He died of radiation exposure within two days.
The Controlled Nuclear ReactorBecause a nuclear reactor requires neutrons that have slowed down, it has a built-in delay and cannot explode like a nuclear bomb (even if scary films claim otherwise). Still, the chain reaction can grow very rapidly, and unless it is controlled, the reactor could in principle heat up to where it melts down. The usual method of control is to insert among the fuel "control rods" which strongly aborb neutrons--e.g. of the metal cadmium, also used in electroplating. By absorbing free neutrons, these rods slow down or stop the chain reaction. Fortunately, nature has been helpful here. About 1% of the neutrons released in fission are not emitted promptly but are delayed, by a fraction of a second. Reactors are always operated to produce just barely enough neutrons to sustain a chain reaction. If for some reason the heat output starts to rise, the delayed neutrons slow down the rate of increase to where an automatic mechanism lowering or raising the control rods is fast enough to stop it. |
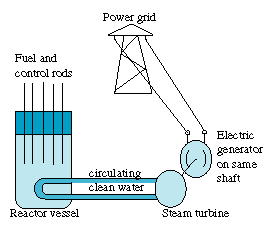
Power reactors in the US use ordinary water as a moderator, inside a "pressure vessel" made of thick steel, with rod-like fuel elements and control rods fitted through openings in its lid. To start the chain reaction Is this the energy of the future? As of this writing (1999) France gets 75% of its energy from nuclear power, and many industrial countries, short on coal and oil, also obtain an appreciable fraction of their energy this way--e.g. 1/3 of the energy used in Japan and Spain. In the US, after an enthusiastic start, the use of nuclear power has leveled off to about 20% of the power generated, mainly due to public resistance to nuclear energy. The US however is fortunate in having large reserves of coal: its growing energy consumption is largely met by these fuels. Environmentally, the choice is between two alternatives:
|
It is not easy to choose, and if we reject both options, we can expect much higher power costs and much less available power. Nuclear WasteThe trouble with fission power is that the "fission fragments" from the break-up of uranium or plutonium are very "hot, " extremely radioactive. This creates two serious problems:
Nuclear AccidentsSuppose something went wrong with the cooling mechanism. Automatically, of course, control rods are lowered and any chain reaction stops immediately. But the radioactive waste in the reactor core continues to generate heat, so that cooling must still be provided for some hours, if not days. On 28 March 1979, at the nuclear power station at Three Mile Island, outside Harrisburg, Pennsylvania, a minor malfunction led to a series of errors, shutting down for a while both the main and emergency cooling systems. The residual heat of the nuclear wastes melted part of the core and created (by chemical reaction) free hydrogen, which further complicated the situation. The billion-dollar reactor was a total loss, but the worst damage was probably to the public's trust in the safety of nuclear power. Still, the reactor vessel was not breached, and the second line of defense, the heavy concrete "containment building" inside which it was housed, also remained intact. The power reactor at Chernobyl, near Kiev, capital of the Ukraine, had a different design--like Fermi's original reactor, it used a pile of carbon (graphite) to slow down neutrons, with pipes inside it carrying the fuel rods, control rods and cooling water. It was a big reactor, and was not enclosed in a containment building. On 25 April 1986, an unwise engineering experiment at low power got out of control. The power level surged, the reactor vessel burst, and the hot steam and graphite (as well as combustible zirconium metal used in the fuel rods) reacted with hot steam and with oxygen of the atmosphere to produce an intense fire, whose plume rose to high altitudes and spread radioactive debris over a wide area. Of the fire-fighters called to extinguish the fire, many later died from radiation. Towns and villages near Chernobyl had to be evacuated (they remain empty as of 1999) and agricultural produce over much of Europe was contaminated. The remains of the reactor were later encased in a thick cover of concrete, entombing the radioactive waste left inside it. Since these accidents, nuclear power generation in the US has proceeded without any major mishap. However, nuclear waste is still kept in temporary storage, as the national policy for its treatment and disposal continues to be debated. The other reactor at at Three Mile Island (and even the ones at Chernobyl) have been restarted to supply power again. As the example of France suggests, nuclear power can be the main energy source of an industrial nation, though it calls for a high level of professional competence and carefully designed safety features. At the same time, the accidents at Three Mile Island and Chernobyl are a reminder that this power source carries unique risks of its own. Further ExploringIf you have the time and the motivation, an enormous number of sources can give you more detailed information about matters discussed here, both in print and on the web. You might start with this rather detailed web site, comparable in size to the entire "Stargazers" collection!
"From Stargazers to Starships" continues with sections dealing with spaceflight and spacecraft, starting with The Principle of the Rocket |
|Astro Net ® Home Page | Kanishkas Home Page|

Copyright © 01/2000 Kanishka H. Lankatillake